Recently, Prof. Dong Haohao’s research team at the State Key Laboratory of Biotherapy of West China Hospital (WCH) published a research paper entitled Cryo-EM structures of lipopolysaccharide transporter LptB2FGC in lipopolysaccharide or AMP-PNP-bound States reveal its transport mechanism on the international journal Nature Communications (I.F.11.878).
The main work of this research was accomplished by Prof. Dong Haohao’s team and the collaborator team led by Prof. Zhang Xing, Director of the Center of Crye-Electron Microscopy, Zhejiang University. Dr. Tang Xiaodi, associate professor of the State Key Laboratory of Biotherapy of SCU, Dr. Chang Shenghai, engineer of the Center of Crye-Electron Microscopy, Zhejiang University, Luo Qinghua, postgraduate student at the State Key Laboratory of Biotherapy of SCU, and Mr. Zhang Zhengyu, research fellow at the School of Pharmacy of Wuhan University, are the co-first authors. Dong Haohao, research fellow at WCH, Prof. Zhang Xing at Zhejiang University and Dong Changjiang at the University of East Anglia, UK are corresponding authors, while the National Key Laboratory of Biotherapy/National Center for Clinical Center of Geriatric Diseases are the first institutes of the paper.
At present, the infection arising from the multi-drug-resistant bacteria or “superbug” poses a major threat to human health. New antibiotics are divided into three categories by the World Health Organization (WHO) as per the degree of urgent needs: medium important, very important and extremely important; among which, new antibiotics development for three Gram-negative bacteria---pseudomonas aeruginosa, enterobacter and baumannii---has been regarded as extremely important. Gram-negative bacteria has a unique outer membrane structure, which contains lipopolysaccharide protective layer that pretend the entry of cytotoxic molecules including antibiotics. Disrupting the synthesis and transfer of the outer membrane lipopolysaccharide therefore has become an important method to target antibiotic resistance problem.
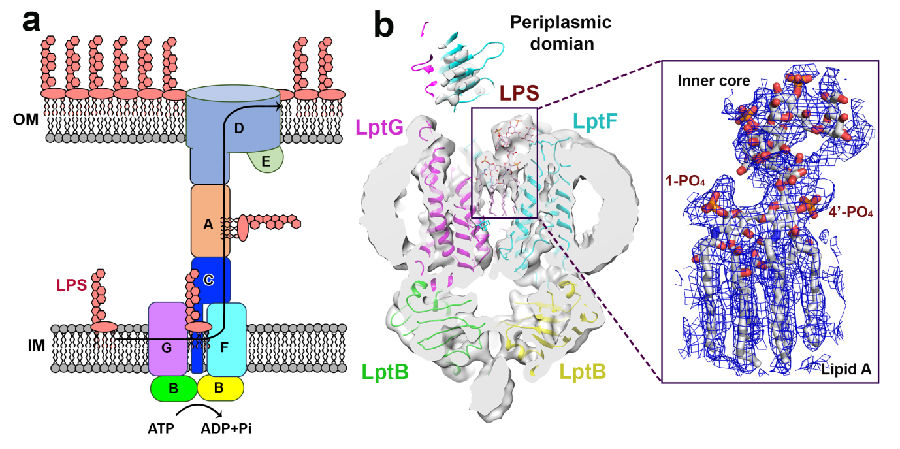
In this study, the target protein machine LptB2FGC in gram-negative bacteria was responsible for the transport of major components of the outer membrane lipopolysaccharide from the inner membrane to the outer membrane. The complex forms a transport channel between the inner and outer membrane, extracting lipopolysaccharide from the inner leaflet of the inner membrane into the transmembrane (TM) substrate binding cavity. ATP binding at the nucleotide binding domain causes conformational changes, which pushes the lipopolysaccharide molecule from the substrate cavity into the periplasmic domain (PD). Finally, lipopolysaccharides are transported by periplasmic transporter proteins to the outer membrane where they form the protective layer. The study revealed the mechanism of LptB2FGC how to recognize and transport LPS, and showed that two arginine rings located at the interface of NBDs and TMDs play an important conformation communication function in transport. In this study, multiple functional amino acids were identified by mutagenesis that are involved in transport and can cause bacterial death. The amino acids can be used as effective targets for novel antibiotic design. The research has important implications for the development of new drugs to fight superbug infectious diseases.